

Introduction of Ion-Molecular Collision:
Charge transfer is a fundamental three-body process throughout the universe, such as the upper atmosphere, interstellar clouds. It is importance to influence the ionic and energy balance in laboratory and astrophysical plasmas [1].
Charge transfer processes between multiply charged heavy-ion Aq+ and molecule,such as H2, include
Aq+ + H2 à A(q-1)+ + H2+ (single capture) ,
Aq+ + H2 à A(q-1)+ + 2H + + e (transfer ionization) , and
Aq+ + H2 à A(q-2)+ + 2H + à A(q-1)+ + 2H + + e
(double-capture autoionization,)
It has been found that ion-molecule charge transfer processes are of particular significance for extreme ultraviolet (EUV) and x-ray emission from comets and from planetary atmospheres. Soft x-ray emission has been observed from many comets, including comet Hale-Bopp [2] (Fig.1, Fig.2 ) and comet Hyakutake [3]. It has been suggested that these EUV and x-ray emissions result from charge transfer of highly charged solar wind ions (e.g., Oq+, Cq+, and Neq+) with cometary neutral species including H2O, its dissociation products H, H2, O, and OH, and CO and CO2 [4,5]. The EUV and x-ray Extreme Ultraviolet Explore (EUE) spectra of comet Hyakutake [6] has revealed emission lines from multiply charged ions (O(4−6)+, C4,5+, and Ne7+). The emission lines due to many highly charged ions including O6+ and O7+ have been -detected in comet McNaught-Hartley by the Chandra X-ray Observatory (CXO). These observations provide strong evidence for the charge transfer mechanism for EUV and x-ray emission from comets. Similarly, observed x-ray emission from the Jovian aurora is thought to be driven by charge transfer in collisions of multiply charged oxygen and sulfur, and possibly sodium and carbon, ions with the atmospheric neutrals H, He, and H2 . The oxygen, sulfur, and sodium ions are thought to be Iogenic and are accelerated into the Jovian atmosphere along closed magnetic field lines connecting the Jovian pole to the outer Io plasma torus. The carbon is believed to be provided by the solar wind .
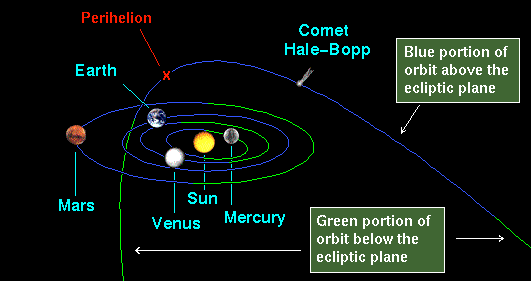
Fig.1
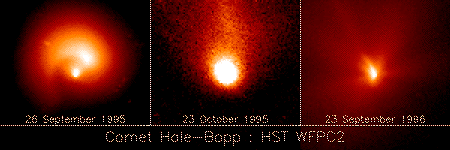
Fig.2
The main research activity of " double star project " (Fig.3 ) undertaking in China, concerns the solar-earth system, namely the celestial body system which direct influence the human activity. These including the upper strata atmosphere of the sun, planetary border space of sun, the earth magnetosphere, ionosphere and atmosphere on the middle and high level of planetary border. The earth space is the area surrounded by the solar wind, and controlled by the earth magnetic field (Fig.4). It is also the main space where various kinds of applied satellites, space station and manned airship operate. In these area, various collision process between the particles of the solar wind and heavy particles occurs, such as the charge transfer processes of heavy particles take place in the aurora area of the two earth poles (Fig.5).
Fig.3
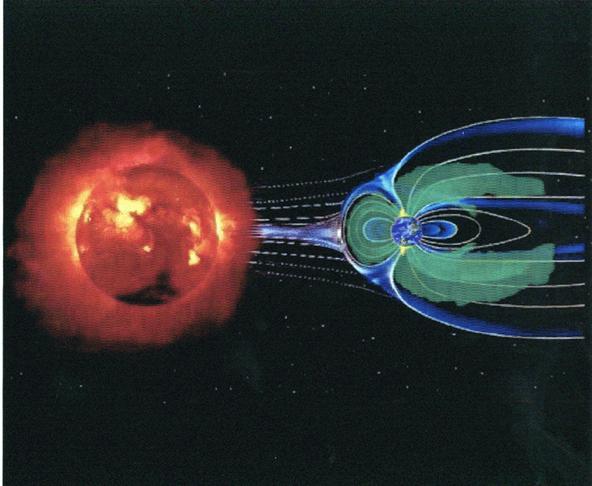
Fig.4

Fig.5
In laboratory plasmas, such as the divertor region of a tokamak fusion device, charge exchange of impurity ions with H2 plays an important role in the ionization balance and its radiative emission is a significant contributor for plasma cooling (Fig.6). This is proved to be due to the charge transfer process between the ionic hydrogen and vibrational excited molecular hydrogen [7].
Fig.6
To simulate the above processes quantitatively, it is essential to obtain the accurate cross section of the charge transfer process, especially the state-selective reaction cross section.
Because of its complexity, charge transfer processes are usually treated as the collision process between the ion and atom target, neglect the vibrational states of the molecular target [8]. At high energy, various method have been employed. These include the Oppenheimer-Brinkman-Kramer (ORK), Born, distorted wave, Glauber, eikonal, impulse, continuum distored wave, continue intermediate states, Fadeev, classical, and refined-orthogonal closed coupling[9]. In 1970s, Olsen et al.[10] proposed an empirical method utilizing Landau-Zener method and combining Frank-Condon factor to deal with the vibrational states of the molecule target, but this method is not so accurate. In the 1980s, Baer et al[11] calculated the charge transfer processes between the ion and molecular hydrogen using fully quantum molecular orbital method, the vibrational states of the molecular target is included. Recently, by using the same method, Krstic et al [12]. calculated the charge transfer processes between the hydrogenic ion and molecule hydrogen, including the dissociate process. In their calculation, the model potential is used to obtain the coupling matrix element, and it is proper for the low energy collision process. Since the end of the 1990s, Errea et al.[13] utilized the semi-classical molecule orbital strong coupling method to deal with the charge transfer processes between the multiply charged ion ion and molecule hydrogen. Similar system is also treated by Phillip et al [14] using full quantum molecule orbital strong coupling method to obtain the vibrational state-selective cross section of the charge transfer process. A recent review on “Charge-transfer dynamics studied using resonant core spectroscopies” can be found on Ref. [15].
[1] D. S. F. Crothers, Adv. In At and Mol. Phys. 17, 55, 1981, and references there in.
[2] A. Owens et al.,Astrophys. J. 493, L47 (1998).
[3] C. M. Lisse et al., Science 274, 205 (1996).
[4] T. E. Cravens, Geophys. Res. Lett. 24, 105 (1997).
[5] R. M. Haberli et al., Science 276, 939 (1997).
[6] V. A. Krasnopolsky et al., Astrophys. J. 549, 629 (2001).
[7] R. K. Janev et al., Phys. Plasma 7, 4364 (2000).
[8] E.g., R. Shingal and C. D. Lin, Phys. Rev. A 40, 1302 (1989);
W. Fritsch and C. D. Lin, Phys. Lett. A 166, 238 (1992);
W. Fritsch and C. D. Lin, J. Phys. B 27,3461 (1994);
T. Kusakabe et al., Phys. Rev. A 62, 062715 (1999);
L. Pichl et al., J. Chem. Phys. 118, 4872 (2003).
[9] Robin Shakeshaft, Larry Spruch, Rev. Mod. Phys. 51, 369 (1979)
[10] R. E. Olsen and A. Salop, Phys. Rev. A 14, 579 (1976).
[11] M. Baer and H. Nakamura, J. Chem. Phys. 87, 4651 (1987); M. Baer et al., J. Chem. Phys. 88,1461 (1987).
[12] P. S. Krstic, Phys. Rev. A 66, 042717 (2002); P. S. Krstic et al., Phys. Rev. A 67, 022708 (2003).
[13] L. F. Errea et al., J. Phys. B 32, 4065 (1999); L. F. Errea et al., J. Phys. B 33, 3107 (2000); L. F. Errea et al., J. Phys. B 33, L615 (2000).
[14] J. G. Wang et al., Vibrationally-resolved charge transfer of O3+ with molecular hydrogen, Phys. Rev. A (in press, 2004).
|
[15] P. A. Brühwiler, O. Karis, and N. Mårtensson, Rev. Mod. Phys. 74, 703 (2002) |
[Close]